
Dr. Jason Pang is an accomplished professional in the field of dentistry, boasting a distinguished academic background. He earned a Bachelor of Science (Biomedical Science) with a University Medal in 1994 from the University of Technology, Sydney, followed by an Honours degree in 1995, during which he conducted research on Cardiac Arrhythmia drugs. In 2002, Dr. Pang furthered his education by completing a Bachelor of Dental Surgery at The University of Sydney. Known as “the Smile Architect”, the expertise of the Neutral Bay dentist in Sydney extends across various dental disciplines, encompassing areas such as teeth whitening, smile makeovers, dental implants, and gum and periodontal treatment. Beyond his dental practice, Jason Pang is the proud proprietor of Mindflux, a center of excellence in dentistry. He is also an accomplished author and a distinguished international speaker. His extensive experience and contributions in the field of dentistry reflect his commitment to advancing the art and science of oral care.
Abstract
Implants can be an excellent alternative to replace lost teeth and their placement could be considered somewhat routine. However, implants are not without their own problems. As implant placement rates increase, so does the number of patients/implants that appear with peri-implant disease.
Studies show that up to 80% of patients may experience some form of inflammatory peri- implant complications [1]. And with no predictable, established way of managing peri- implantitis, primary prevention and early management is key [2].
Introduction
It is well-accepted that inflammation and a dysbiotic polymicrobial community are associated with periodontal and peri-implant disease.
However, more and more evidence is showing that peri-implant disease is not periodontal disease3,4. There are different early colonisers on the implant surface compared to the cementum.
It is similar in that it is an immune-mediated inflammatory process in the tissues surrounding implants in the presence of predominantly bacterial biofilms on the surface of the implant. What remains unclear is whether the inflammation precedes and allows the bacteria to overgrow and gain entry to the epithelium or if the dysregulated overgrowth (dysbiosis) itself causes the inflammation5.
Regardless, it is when the two conditions co-exist that we see the clinical signs of inflammation around an implant.
Polymicrobial synergy and dysbiosis occur in clusters where ecologic factors and interbacterial interactions cause an overgrowth of pathobionts.
This leads to:
• Increased expression of virulence factors;
• Dysregulation of immune surveillance/response and
• Disruption of tissue homeostasis
Presentation of peri-implant disease
• Implant mucositis 1.
– up to ~80% of implants
– reversible gingival inflammation
– erythema, swelling & bleeding of soft tissues surrounding an implant
• Peri-implantitis 1,2
– around 14-30% of implants
– dysregulated inflammation
– tissue damage around an implant, resulting in the loss of the supporting bone
Rate and progression of peri-implant disease
• Peri-implant mucositis is the precursor to peri-implantitis, as is gingivitis for periodontitis – a continuum exists2
• Peri-implantitis (PI) progresses in a non-linear, accelerating pattern and in the majority of cases, onset occurs within 3 years of function6,7
• No effective treatment of PI exists
– peri-implant mucositis management is considered a preventive measure for the onset of peri-implantitis 2
Risk factors for peri-implant disease
• Inconclusive evidence
– diabetes 6
• Limited evidence – areas of future research
– submucosal cement, keratinized mucosa and implant position, occlusal factors, systemic conditions 6
• Moderate evidence – smoking 8
• Strong evidence
– poor plaque control, irregular maintenance therapy, and chronic periodontal disease 6
Despite the prevalence, diagnosing peri-implant disease remains challenging as common diagnostic methods of periodontal probing and radiographs may be inaccurate. These methods only document pre-existing destruction rather than current disease activity.
Periodontal classification uses staging and grading to assess disease progression. Staging measures the severity and distribution of damage. Grading attempts to predict or measure disease activity but has not been developed past an assessment of risk factors 9.
Biomarkers
Salivary biomarkers of the host and the microbial flora are being looked at to differentiate those who are periodontally healthy and those that have disease. Host biomarkers such as Receptor Activator of Nuclear factor Kappa-B Ligand (RANKL) 10,11, osteoprotegerin (OPG) 11, matrix metalloproteinases eg. MMP-9 12and genetic markers like interleukins eg. IL- 6 12, IL-23 10, IL-1β 12,13, and tumour necrosis factor α 13 can all be helpful in determining the change from peri-implant health to peri-implantitis.
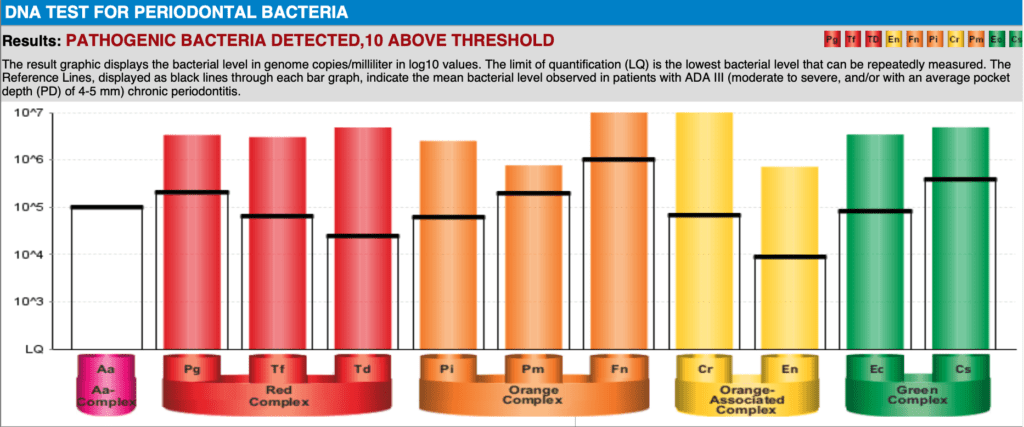
However, current research is still insufficient to determine PI progression with any certainty. Moreover, these tests are costly and time-consuming and may not be sufficient motivation to encourage the patient to change their lifestyle and home oral hygiene habits. Microbial biomarkers from saliva can also be informative to determine those who have disease. Bacteria can be categorised into complexes based on their relative risk. Bacteria such as Aggregatibacter actinomycetemcomitans (A.a) and the ‘red complex’ (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) are considered to put an individual at the highest risk of periodontal disease when their numbers exceed threshold levels 5,14–16. Similarly in peri-implantitis, ‘red complex’ are found in higher numbers than in healthy patients along with Staphylococcus aureus and other uncultivatable bacteria 15,17. DNA PCR testing can yield quantitative data on the periodontal bacteria that are present in the oral cavity (Fig. 1).
However, this test is costly, only available overseas with results taking several weeks. It can provide actionable information about risk prior to implant placement but it is much more difficult to determine when to retest as periodontal bacteria are notoriously resistant. Several rounds of testing will become very costly for the patient.AL REPORT
The taxonomic composition of the oral microbiome (OM) in periodontally healthy individuals can be biased because the clinically periodontally healthy subjects for evaluation The taxonomic composition of the oral microbiome (OM) in periodontally healthy individuals can be biased because the clinically periodontally healthy subjects for evaluation can already experience dysbiosis.
In many studies, subjects are considered healthy when there is an absence of clinical signs of periodontitis18. It is important to note that the dysbiosis of the OM occurs before the manifestation of clinical symptoms, sometimes months or even years in advance. Therefore, the absence of periodontal pockets does not necessarily indicate good periodontal health, and the perception of a “healthy OM” can be misleading or distorted18.
The OM has the second most diverse microbial population of the body, after the gut microbiome. Over 775 species are known to colonise the mouth, with individuals usually having 200-300 species each19,20. Most of the bacteria exist in biofilms on the various surfaces of the mouth including teeth, gum, periodontal pockets, tongue, palate, dentures, as well as other non-removable structures like crowns, bridges and implants. The bacteria are considered commensal when they help to maintain homeostasis and prevent overgrowth of any particular bacteria.
Opportunistic microbiota or pathobionts can be bacterial, fungal, yeast, viral or parasitic in decreasing order. With poor oral hygiene, slower-growing bacteria attach to the faster-growing bacteria and create an environment for more pathogenic bacteria. The thicker and more mature biofilm form a complex environment that creates an imbalance in the OM known as dysbiosis. The toxins and enzymes released can create an inflammatory state where clinical signs of inflammation are then visible. The inability to cultivate and characterize many of the oral taxa makes it difficult to determine the microbiota associated with peri-implantitis.
However, with newer molecular detection methods such as 16S ribosomal RNA-based metabarcoding, whole metagenome shotgun sequencing or meta-transcriptomics the bacteria involved are becoming clearer, but this is not without cost, time and meticulous sampling. A tool used in the 1980s, that was of enormous benefit to dentists at the time, was the phase-contrast microscope21,22. Phase-contrast microscopy (PCM) exploits the refractive index differences between the microorganisms and their surroundings to enhance the contrast and allow for better visualization of their morphology and movement.
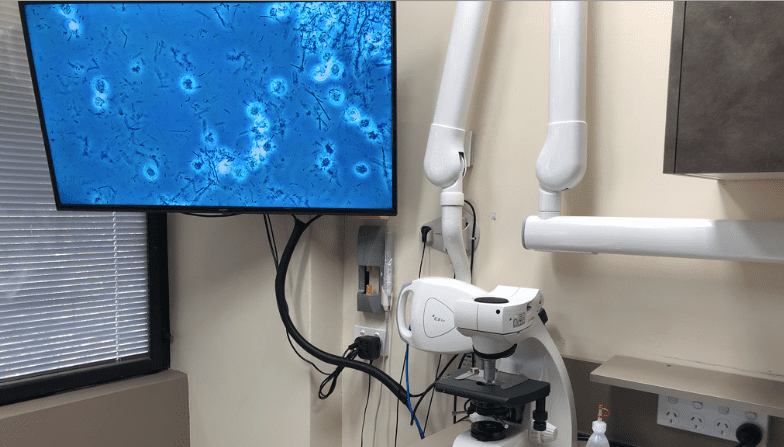
It allowed the dentist to visualize the patient’s bacteria but the patient was not involved in the screening process. By connecting the PCM image to large format screens, the patient can see the bacteria from their mouths, at 1000x magnification and be educated as to the bacterial load, motility and morphotype. This real-time observation of their oral flora dramatically increases engagement and can encourage more optimal home oral hygiene protocols (Fig. 2).
In 2015, using PCM Rams and Keyes23 found that the presence of subgingival spirochetes and crevicular leukocytes could act as a simplified biomarker for dysbiosis and host inflammatory response and diagnostically useful for assessing the risk of progressive disease in chronic periodontitis patients.
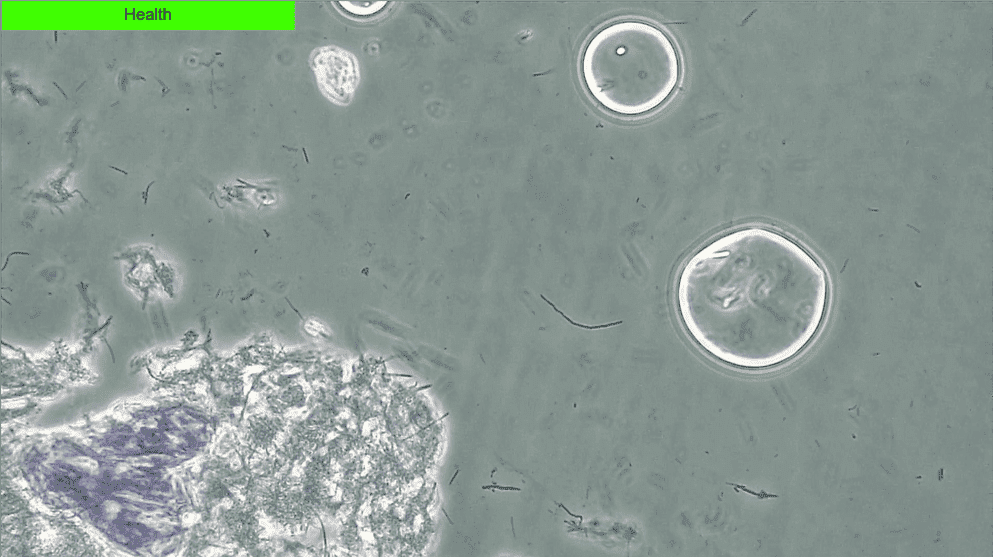
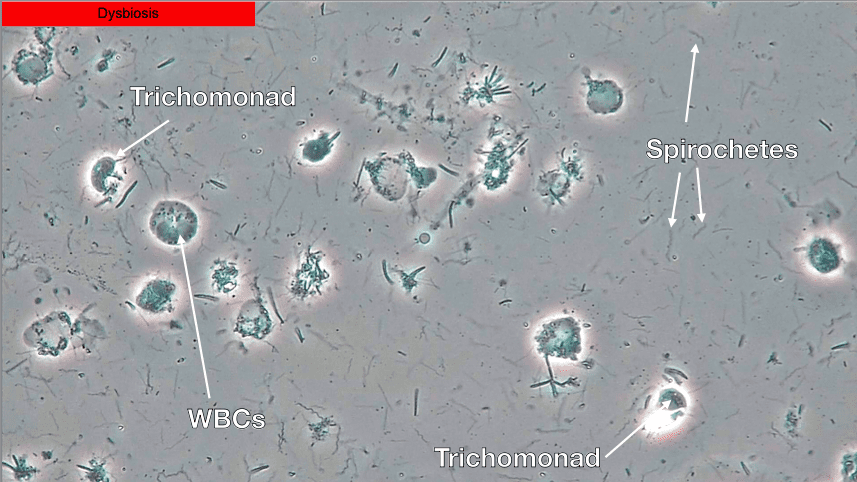
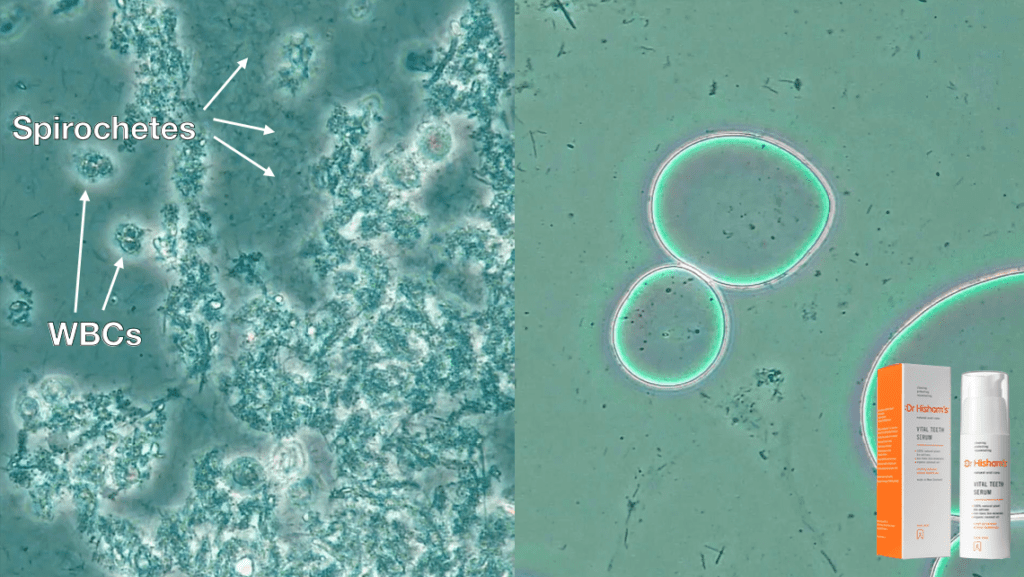
Though it was a small study, the 100% negative predictive value associated with PCM suggests that if no or low spirochete and crevicular leukocyte counts are attained then the risk of chronic periodontal disease progression is minimal23. Healthy biofilms are very still and have an absence of spirochetes and white blood cells (WBCs) (Fig. 3) while dysbiotic biofilms will show a lot of activity with spirochetes, WBCs and motile bacteria (Fig. 4).
Many different species of spirochetes exist and they are diverse in their pathogenic capacity, the most infamous being Treponema pallidum which causes syphilis. Treponema denticola is an oral spirochete and has consistently remained a microbial biomarker of interest for both periodontal and peri-implant disease15,16,24–31. Spirochetes have a unique spiral-shape compared with spheres (cocci) and rods (bacilli) and other irregular shapes. Their movement is distinct and allows them to burrow into epithelial tissues27.
In viewing slides of patients with deep periodontal pocketing, we have yet to see one that does not have spirochetes as the dominant morphotype in the sample. For peri-implant mucositis and peri- implantitis (PI) we often see mixed biofilms with high numbers of cocci and rods and variable amounts of spirochetes32–35. Subgingival samples taken from teeth adjacent to healed extraction sockets will provide information about the number of spirochetes and the potential risk of peri- implant complications.
In a 10-year retrospective study of peri-implantitis on rough surface implants, Caccianiga et al.36 found that by evaluating subgingival biofilm with PCM every 4 months they could screen patients for dysbiosis and commence treatment prior to clinical signs of PI. By performing treatment with a diode laser with stabilised H2O2 they were able to minimise PI to 1.5% of implants, losing only 4% of implants.
Using PCM, dysbiosis and peri-implant mucositis can be detected prior to the progression to PI. Early detection means early treatment with non-surgical options. Disclosing solution applied to the teeth allows observation of mature biofilm for improved home hygiene measures. Professional biofilm removal can be performed with non-abrasive air polishing. In studies of PI, air-polishing with erythritol has shown superior or similar clinical outcomes to mechanical debridement with manual instruments. These results were substantiated by the reduction in the microbial load as well as the reduction in the inflammatory cytokines37,38.
Lasers can be a useful adjunct to root surface debridement as they allow deep and lasting decontamination of pathogens, removal of infected epithelium and inactivation of bacterial endotoxins in periodontal pockets and implant surfaces 39,40. High powered diode lasers can be used for laser bacterial reduction (LBR), in combination with photosensitisers to release oxygen free radicals in photodynamic therapy (PDT) or in combination. Wavelengths such as Er:YAG and Nd:YAG can denature microbiota and remove diseased biofilm41–43. Many bacteria are resistant to conventional debridement as A.a., P. gingivalis, T. forsythia, F. nucleatum, P. intermedia and T. denticola can all internalize within epithelial cells44,45.
Using lasers may be of benefit as a minimally invasive method of de- epithelialising the deep infected pockets compared with root surface debridement alone41–43. The use of Er:YAG laser to create photoacoustic shockwaves for biofilm removal from the implant surface also shows merit46–49. Antimicrobial agents that can assist in the decontamination of the implant surface can help to maintain successful osseointegration. Ozone is a powerful oxidant that is used worldwide for water purification due to its antibacterial, antiviral and antifungal properties. It is a tri-oxygen molecule that can be applied in gaseous or aqueous form. Ozone has rapid- acting properties to damage prokaryotic cells without antioxidant defenses. In human cells, it is also anti-inflammatory, stimulates wound healing and activates the cellular and humeral immune system50,51.
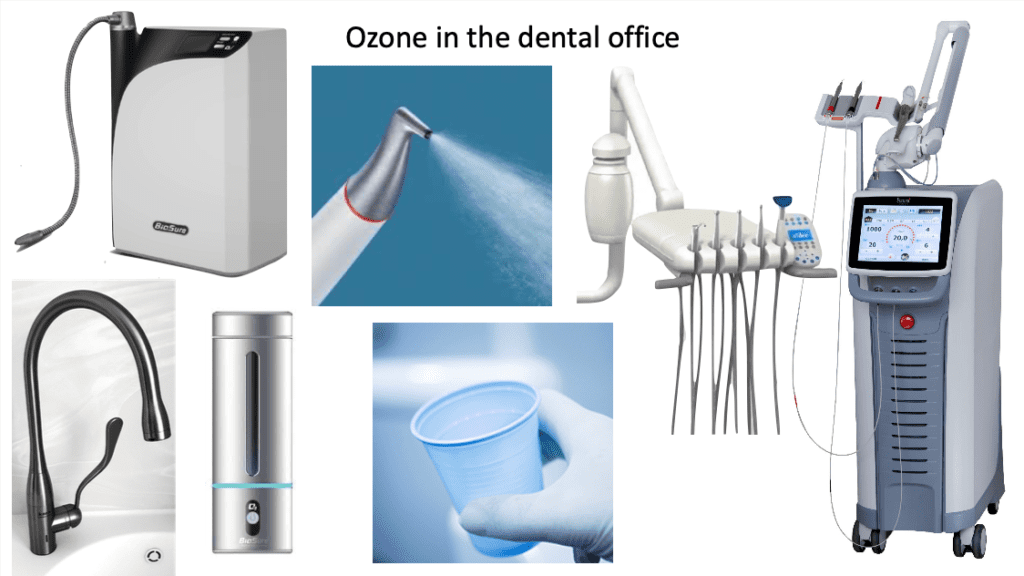
In dentistry, we use ozonated water to manage biofilm in dental water lines, as a pre-operative rinse and it can be used in our air polishing equipment and lasers for active disinfection during treatment52. It can be dispensed at various concentrations (1-4 ppm) from taps or through rechargeable portable devices for home use (Fig. 5). Patients report reduced bleeding, sensitivity and gum problems from daily use. Dramatic changes in patients’ OM have been observed in as little as two months of regular use.
Mature subgingival microbiota can be observed within a week so regular and accurate disruption of the implant surface is essential to prevent peri-implant complications. Combination therapy of ozone in air polishing and laser devices shows rapid changes to biofilm and prevention of recolonisation of spirochetes.
Parasites
Parasites such as protozoa or helminths (worms) can be found in the oral cavity and may contribute to oral infections or diseases. The two most common oral protozoa are Entamoeba gingivalis and Trichomonas tenax. They are considered commensal and believed to be more prevalent with poor oral hygiene and lower socioeconomic areas however that belief may be changing53.
Several studies link the presence of amoeba with not only periodontal disease but associating it with other chronic health conditions like diabetes and hypertension54–57. The higher prevalence of parasites in periodontal disease may elevate its status to being more than a mere marker for the disease. Biofilms with parasites are easily distinguishable due to their movement and irregularly- shaped WBCs. Clinical signs of inflammation are frequently also present when these parasites are visible. The removal of these parasites through optimal home hygiene protocols is problematic and the use of antiparasitic antibiotics such as metronidazole could improve outcomes58,59.
Case Report 1
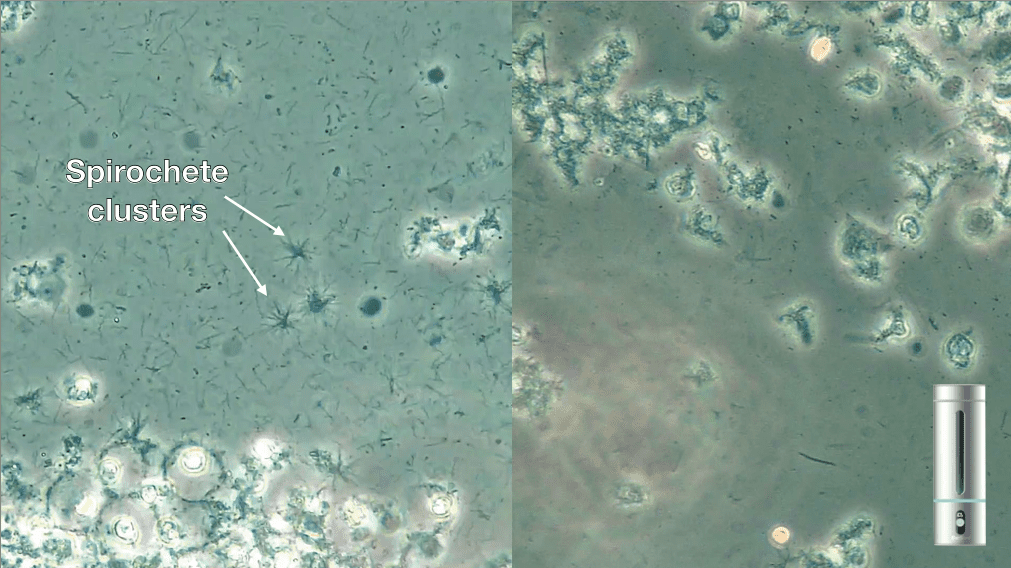
A 70-year old patient presented to our clinic after a recent heart attack where he had three stents placed. This was during a period when COVID-19 was considered high-risk and no hygiene procedures were being performed. His bacterial screening showed a high level of dysbiosis with clusters of spirochetes despite routine bi-daily brushing and flossing. As we were unable to perform any professional biofilm removal we advised him to use a portable ozonated water generator to ozonate water to rinse with for 60s after brushing. Within 3 months, we can see that the number of spirochetes had dramatically decreased and there were minimal signs of dysbiosis (Fig. 6).
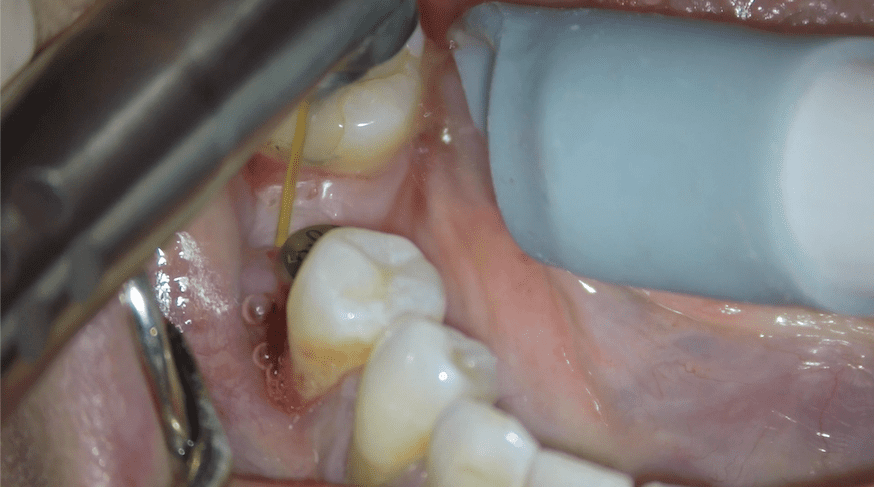
Case Report 2
A 26-year old female had a bone level implant and healing abutment placed into a healed 36 position. Two months later the patient presented with implant mobility, suppuration and bleeding. Her oral biofilm showed moderately-high motility bacteria and WBCs and high numbers of spirochetes. Treatment involved Guided Biofilm Therapy (GBT) and Laser Assisted Peri-Implant Treatment (LAPIT) (Fig. 7) and torquing the implant to 35Nm. The patient maintained immaculate oral hygiene with Dr Hisham’s Vital Tooth Serum (coconut oil, xylitol, totarol oil) and 5 weeks later showed no clinical signs of inflammation, low bacterial load and low motility bacteria with minimal spirochetes. A one-month review showed no complications and the implant was successfully restored at 6 months post- placement (Fig. 8). No signs of dysbiosis were present at the time of implant crown placement.
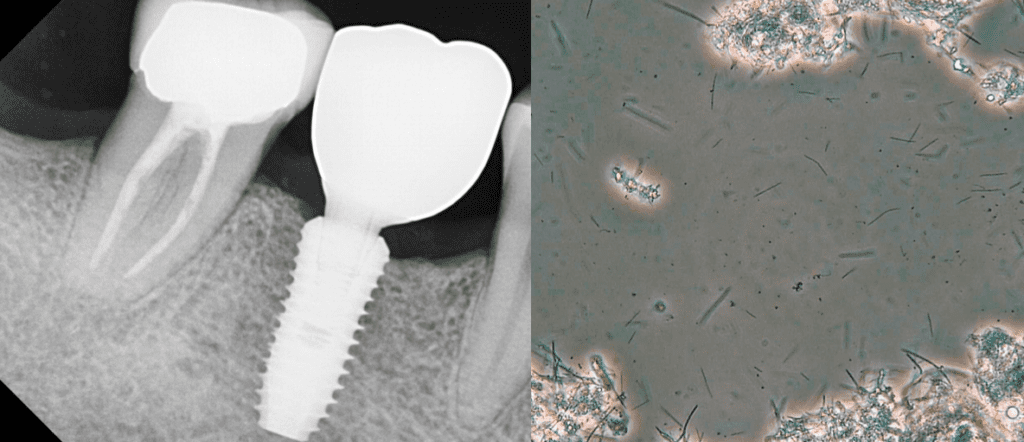
Case Report 3
A periodontal patient had a healed extraction socket with bone augmentation. Disclosing solution showed average competency of biofilm removal. Using PCM, we assessed the biofilm and found a moderate bacterial load with low- moderate motility and moderate-high numbers of spirochetes. As the patient was highly motivated to replace his lost tooth with an implant he commenced daily ozone rinsing.
Two months later we saw a low bacterial load with low motility and no visible spirochetes. An implant was placed in the 46 position and showed no clinical signs of inflammation and continued to show no signs of dysbiosis of his biofilm. DNA PCR testing showed that 4 of the 11 periodontal bacteria were present but at below threshold levels indicating stable periodontal health. The implant was restored 15 months after placement and continued to show good periodontal health and a healthy biofilm (Fig. 9).
Case Report 4
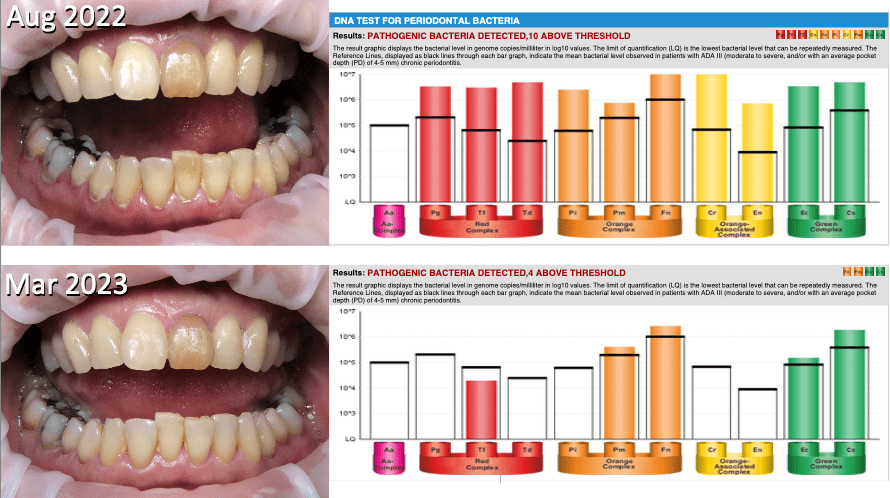
A 54-year old presented with class 2 mobility of the 16 and bone loss around many molars. The patient would be classified as Stage 4, Grade C, unstable, generalised periodontitis. As the risk for implant placement is chronic periodontal disease, the patient does not want to risk placing an implant when there is a high risk of failure. Treatment included extraction of the 16, ozone with GBT and PCM assessment.
Her biofim showed extreme dysbiosis with high bacterial load with high motility bacteria and severe spirochetosis. With daily rinsing of Colgate Peroxyl, three monthly ozone with GBT a dramatic improvement was seen. DNA PCR test at presentation showed 10 of 11 pathogenic bacteria present above threshold and 7 months later after two rounds of GBT showed 5 bacteria present with 4 above threshold (Fig. 10). After 10 months, there was no BOP and only 3mm pocketing. The patient was advised that implant placement now had a high confidence level of success but regular maintenance and OM monitoring was essential.
Case Report 5
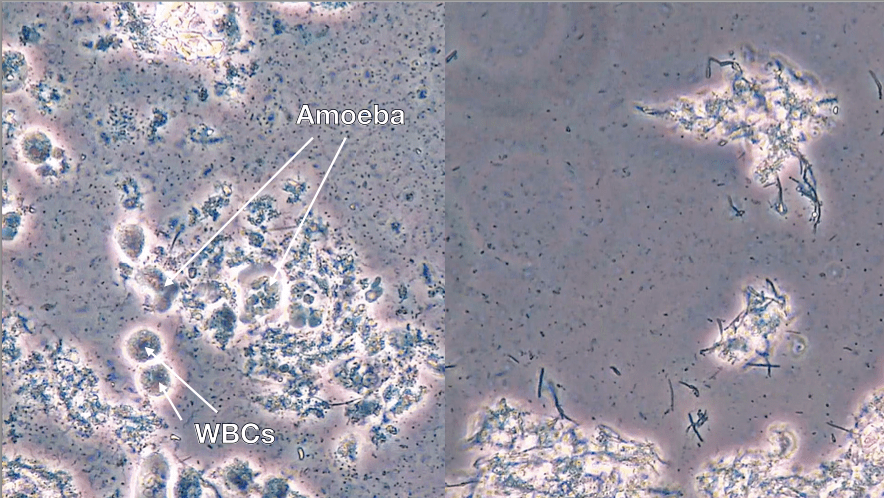
A stable periodontal patient with an implant placed 10 years ago showed rapid bone loss, bleeding and suppuration i.e. PI. Upon assessment of their biofilm with PCM we found a high bacterial load with moderate motility and high numbers of spirochetes together with the presence of a parasite i.e. high numbers of amoeba. Ozone with GBT and Amoxycillin/ Metronidazole antibiotics were prescribed. Two-week follow-up showed moderate- high load and low-moderate motility but an absence of spirochetes and amoeba (Fig. 11). Regular maintenance therapy and exceptional oral hygiene will be required to prevent a relapse.
In summary PCM is a quick, easy and inexpensive way to determine:
• When there is oral dysbiosis and inflammation
• If a patient’s home care regime is sufficient
– are they compliant or is additional home care equired?
• If our therapy has caused a bacterial/ inflammatory shift
– has bacterial load/motility reduced and their morphotype changed?
– have leukocyte numbers reduced and their morphotype changed?
• When retreatment/alternative treatment is necessary
Conclusion:
Optimal management of the OM for peri- implant disease will require:
• Excellent oral hygiene
• Regular professional biofilm monitoring and removal
– DNA PCR testing may be of benefit
When phase-contrast microscopy shows a dysbiotic biofilm with or without clinical signs of inflammation this may require:
• Improved oral hygiene and adjuncts to cleaning
– oral irrigator, interproximal brushes, tongue scraping
• More frequent professional biofilm removal
• Antimicrobial rinses
– ozonated water, H2O2, peroxyl, chlorhexidine etc.
• Antiparasitic antibiotics for parasites u Laser therapy and/or surgery DRA
World Federation of Laser Dentistry slide presentation on this topic with video of live biofilm can be viewed at: https://youtu.be/M1DnZNTwdqo
References
1. Bäumer A, Toekan S, Saure D, Körner G. Survival and success of implants in a private periodontal practice: A 10 year retrospective study. BMC Oral Health. 2020;20(1):1– 10.
2. Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J Clin Periodontol. 2015;42(S16):S152–7.
3. Kalsi AS, Moreno F, Petridis H. Biomarkers associated with periodontitis and peri- implantitis: a systematic review. J Periodontal Implant Sci. 2021;51(1):3–17.
4. Kotsakis GA, Olmedo DG. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol 2000. 2021;86(1):231–40.
5. Van Dyke TE, Bartold PM, Reynolds EC. The Nexus Between Periodontal Inflammation and Dysbiosis. Front Immunol. 2020;11(March):1–9.
6. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018;45(June 2016):S246–66.
7. Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Peri- implantitis – Onset and pattern of progression. J Clin Periodontol. 2016;43(4):383–8.
EDITOR’S PAGE | ADVISORY BOARD | NEWS | PRODUCTS | FEATURE ARTICLE | CLINICAL | PROFILE | EXHIBITIONS & CONFERENCES | PRODUCT TIPS | DENTAL BUSINESS
8. Reis INR dos, do Amaral GCLS, Hassan MA, Villar CC, Romito GA, Spin-Neto R, et al. The influence of smoking on the incidence of peri-implantitis: A systematic review and meta-analysis. Clin Oral Implants Res [Internet]. 2023 Jun 28;34(6):543–54. Available from: https://onlinelibrary.wiley.com/doi/10.1111/clr.14066
9. Gellibolian R, Miller CS, Markaryan AN, Weltman RL, Van Dyke TE, Ebersole JL. Precision periodontics. J Am Dent Assoc [Internet]. 2022 May;1–3. Available from: https://doi.org/10.1016/j.adaj.2022.03.005
10. Chaparro A, Beltrán V, Betancur D, Sam YH, Moaven H, Tarjomani A, et al. Molecular Biomarkers in Peri-Implant Health and Disease: A Cross-Sectional Pilot Study. Int J Mol Sci. 2022;23(17).
11. Rakic M, Struillou X, Petkovic-Curcin A, Matic S, Canullo L, Sanz M, et al. Estimation of Bone Loss Biomarkers as a Diagnostic Tool for Peri-Implantitis. J Periodontol. 2014;85(11):1566–74.
12. Zhang X, Wang Z, Hu L, Shen X, Liu C. Identification of Potential Genetic Biomarkers and Target Genes of Peri-Implantitis Using Bioinformatics Tools. Biomed Res Int. 2021;2021.
13. Alassy H, Parachuru P, Wolff L. Peri-implantitis diagnosis and prognosis using biomarkers in peri-implant crevicular fluid: A narrative review. Diagnostics. 2019;9(4).
14. Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. Segata N, editor. J Clin Periodontol [Internet]. 2019 Jan 27;46(1):6–11. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jcpe.13046
15. Wang HL, Garaicoa-Pazmino C, Collins A, Ong HS, Chudri R, Giannobile W V. Protein biomarkers and microbial profiles in peri-implantitis. Clin Oral Implants Res [Internet]. 2016 Sep;27(9):1129–36. Available from: https://onlinelibrary.wiley.com/doi/10.1111/clr.12708
16. Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and treponema denticola in subgingival plaque. Oral Microbiol Immunol. 2009;24(6):469– 77.
17. Charalampakis G, Belibasakis GN. Microbiome of peri-implant infections: Lessons from conventional, molecular and metagenomic analyses. Virulence [Internet]. 2015 Apr 3;6(3):183–7. Available from: http://www.tandfonline.com/doi/full/10.4161/21505594.2014.980661
18. Lenartova M, Tesinska B, Janatova T, Hrebicek O, Mysak J, Janata J, et al. The Oral Microbiome in Periodontal Health. Front Cell Infect Microbiol [Internet]. 2021 Mar 22;11(March). Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2021.629723/full
19. Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML, et al. The oral microbiome – An update for oral healthcare professionals. Br Dent J. 2016;221(10):657–66.
20. Human Oral Microbiome Database [Internet]. [cited 2023 Mar 1]. Available from: http://www.homd.org
21. Keyes PH, Rams TE. A Rationale for Management of Periodontal Diseases: Rapid Identification of Microbial ‘Therapeutic Targets’ with Phase-Contrast Microscopy. J Am Dent Assoc [Internet]. 1983 Jun;106(6):803–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002817783660172
22. Rams TE, Keyes PH, Wright WE. Treatment of juvenile periodontitis with microbiologically modulated periodontal therapy (Keyes technique). Pediatr Dent. 1985;7(4):259–70.
23. Keyes PH, Rams TE. Subgingival Microbial and Inflammatory Cell Morphotypes Associated with Chronic Periodontitis Progression in Treated Adults. J Int Acad Periodontol [Internet]. 2015 Apr;17(2):49–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26242011
24. Riviere GR, DeRouen TA, Kay SL, Avera SP, Stouffer VK, Hawkins NR. Association of Oral Spirochetes From Sites of Periodontal Health With Development of Periodontitis. J Periodontol [Internet]. 1997 Dec;68(12):1210–4. Available from: https://onlinelibrary.wiley.com/doi/10.1902/jop.1997.68.12.1210
25. Pisani F, Pisani V, Arcangeli F, Harding A, Singhrao SK. Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer’s Disease? Int J Environ Res Public Health. 2023;20(2).
26. Hong BY, Araujo MVF, Strausbaugh LD, Terzi E, Ioannidou E, Diaz PI. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS One. 2015;10(5):1–14.
27. Pisani F, Pisani V, Arcangeli F, Harding A, Singhrao SK. The Mechanistic Pathways of Periodontal Pathogens Entering the Brain: The Potential Role of Treponema denticola in Tracing Alzheimer’s Disease Pathology. Int J Environ Res Public Health. 2022;19(15).
28. Simonson LG, Robinson PJ, Pranger RJ, Cohen ME, Morton HE. Treponema denticola and Porphyromonas gingivalis as Prognostic Markers Following Periodontal Treatment . J Periodontol. 1992;63(4):270–3.
29. Belibasakis GN, Mir-Mari J, Sahrmann P, Sanz-Martin I, Schmidlin PR, Jung RE. Clinical association of Spirochaetes and Synergistetes with peri-implantitis. Clin Oral Implants Res [Internet]. 2016 Jun;27(6):656–61. Available from: https://onlinelibrary.wiley.com/doi/10.1111/clr.12690
30. Meffert RM, Antonio S. Periodontitis Vs . Peri-implantitis : The same disease? The same treatment? Crit Rev Oral Biol Med. 1996;7(1985):278–91.
31. Simonson LG, Goodman CH, Bial JJ, Morton HE. Quantitative relationship of
Treponema denticola to severity of p eriodontal disease. Infect Immun.
1988;56(4):726–8.
32. Chang JW, Bi J, Owen G, Shen Y, Haapasalo M, Wiebe C, et al. Scanning electron
microscopic analysis of adherent bacterial biofilms associated with peri-implantitis.
Clinical and Experimental Dental Research. 2023.
33. Shibli JA, Melo L, Ferrari DS, Figueiredo LC, Faveri M, Feres M. Composition of supra-
and subgingival biofilm of subjects with healthy and diseased implants. Clin Oral
Implants Res. 2008;19(10):975–82.
34. Dabdoub SM, Tsigarida AA, Kumar PS. Patient-specific analysis of periodontal and
peri-implant microbiomes. J Dent Res. 2013;92(12).
35. Mombelli A, Décaillet F. The characteristics of biofilms in peri-implant disease. J Clin
Periodontol. 2011;38(SUPPL. 11):203–13.
36. Caccianiga G, Rey G, Caccianiga P, Leonida A, Baldoni M, Baldoni A, et al. Rough
dental implant surfaces and peri-implantitis: Role of phase-contrast microscopy, laser protocols, and modified home oral hygiene in maintenance. A 10-year retrospective study. Appl Sci. 2021;11(11).
37. Jentsch HFR, Flechsig C, Kette B, Eick S. Adjunctive air-polishing with erythritol in nonsurgical periodontal therapy: a randomized clinical trial. BMC Oral Health [Internet]. 2020;20(1):1–9. Available from: https://doi.org/10.1186/s12903-020- 01363-5
38. Shrivastava D, Natoli V, Srivastava KC, Alzoubi IA, Nagy AI, Hamza MO, et al. Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy ( GBT ): A Review. 2021;
39. Mizutani K, Aoki A, Coluzzi D, Yukna R, Wang CY, Pavlic V, et al. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000. 2016;71(1):185– 212.
40. Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68(1):217–69.
41. Korosec B, Koron N, Bozic Z. Research Study : Periodontal Tissue Regeneration Following Er : YAG and Nd : YAG Laser Treatments. 2017;2017(1):14–8.
42. Sağlam M, Köseoğlu S, Taşdemir, Erbak Yılmaz H, Savran L, Sütçü R. Combined application of Er:YAG and Nd:YAG lasers in treatment of chronic periodontitis. A split- mouth, single-blind, randomized controlled trial. J Periodontal Res. 2017;52(5):853– 62.
43. Grzech-Leśniak K, Sculean A, Gašpirc B. Laser reduction of specific microorganisms in the periodontal pocket using Er:YAG and Nd:YAG lasers: a randomized controlled clinical study. Lasers Med Sci. 2018;33(7):1461–70.
44. Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000 [Internet]. 2010 Feb;52(1):68–83. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0757.2009.00323.x
45. Inagaki S, Kimizuka R, Kokubu E, Saito A, Ishihara K. Treponema denticola invasion into human gingival epithelial cells. Microb Pathog [Internet]. 2016 May;94:104–11. Available from: http://dx.doi.org/10.1016/j.micpath.2016.01.010
46. Li L. The clinical efficacy of er:Yag lasers in the treatment of peri-implantitis: A systematic review and meta-analysis. Ann Palliat Med. 2021;10(8):9002–14.
47. Kotsakis GA, Konstantinidis I, Karoussis IK, Ma X, Chu H. Systematic Review and Meta-
Analysis of the Effect of Various Laser Wavelengths in the Treatment of Peri-
Implantitis. J Periodontol. 2014;85(9):1203–13.
48. Romanos GE, Javed F. Laser therapy is safe but not superior to conventional
treatment of peri-implantitis. J Evid Based Dent Pract [Internet]. 2015;15(2):55–7.
Available from: http://dx.doi.org/10.1016/j.jebdp.2015.03.005
49. Jezeršek M, Molan K, Terlep S, Levičnik-Höfferle Š, Gašpirc B, Lukač M, et al. The
evolution of cavitation in narrow soft-solid wedge geometry mimicking periodontal
and peri-implant pockets. Ultrason Sonochem. 2023;94(November 2022).
50. Salve A, Ansari S, Gattani D, Kar N, Heda P. Ozone – A versatile therapy in implant
dentistry. J Glob Oral Heal. 2023;5(January):124–7.
51. Deepthi R, Bilichodmath S. Ozone therapy in periodontics: A meta-analysis. Contemp
Clin Dent [Internet]. 2020;11(2):108. Available from:
https://journals.lww.com/10.4103/ccd.ccd_79_19
52. Walsh LJ. Ozone in water: Let the Evidence Speak. Australasian Dental Practice.
2021;May/June:88–95.
53. Bonner M, Fresno M, Gironès N, Guillén N, Santi-Rocca J. Reassessing the Role of
Entamoeba gingivalis in Periodontitis. Front Cell Infect Microbiol. 2018;8(October):1–
10.
54. Fadhil Ali Malaa S, Abd Ali Abd Aun Jwad B, Khalis Al-Masoudi H. Assessment of
Entamoeba Gingivalis and Trichomonas Tenax in Patients with Chronic Diseases and
its Correlation with Some Risk Factors. Arch Razi Inst. 2022;77(1):77–82.
55. Trim RD, Skinner MA, Farone MB, Dubois JD, Newsome AL. Use of PCR to detect
Entamoeba gingivalis in diseased gingival pockets and demonstrate its absence in
healthy gingival sites. Parasitol Res. 2011;109(3):857–64.
56. Yaseen A, Mahafzah A, Dababseh D, Taim D, Hamdan AA, Al-Fraihat E, et al. Oral
Colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-Based Study in Health, Gingivitis, and Periodontitis. Front Cell In fect Microbiol. 2021;11(December):1–16.
57. Ghabanchi J, Zibaei M, Afkar Md, Sarbazie A. Prevalence of oral Entamoeba gingivalis and Trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, southern Iran. Indian J Dent Res [Internet]. 2010;21(1):89. Available from: http://www.ijdr.in/text.asp?2010/21/1/89/62821
58. Heitz-Mayfield LJA. Systemic antibiotics in periodontal therapy. Aust Dent J. 2009;54:S96–101.
59. Leitsch D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology. 2019;146(9):1167–78.
The information and viewpoints presented in the above news piece or article do not necessarily reflect the official stance or policy of Dental Resource Asia or the DRA Journal. While we strive to ensure the accuracy of our content, Dental Resource Asia (DRA) or DRA Journal cannot guarantee the constant correctness, comprehensiveness, or timeliness of all the information contained within this website or journal.
Please be aware that all product details, product specifications, and data on this website or journal may be modified without prior notice in order to enhance reliability, functionality, design, or for other reasons.
The content contributed by our bloggers or authors represents their personal opinions and is not intended to defame or discredit any religion, ethnic group, club, organisation, company, individual, or any entity or individual.

