This article first appeared in Dental Update and is reproduced with permission from Mark Allen Publishers (UK).

DSc, DDS, FRCPath, FDS RCS(Edin), FRACDS Professor Emeritus and Immediate-past Dean; Faculty of Dentistry, University of Hong Kong, Hong Kong.
email: lakshman@hku.hk

PhD, MSc, BDS, SFHEA, FPFA Senior Lecturer, Restorative Dentistry, UWA Dental School, University of Western Australia, Perth, Western Australia, Australia.

BDS, MDS, MFDS RCPS (Glas), Clinical Assistant Professor, Endodontics, Faculty of Dentistry, University of Hong Kong, Hong Kong.
Abstract
Routine vaccination against a number of communicable infectious diseases is an integral part of the infection control regimen of all dental health care workers. The COVID-19 pandemic, now endemic in many regions of the World, and the multiple vaccines that have been introduced in the wake of this illness have once again brought into focus this important issue.
The immunogenicity, durability of vaccine-induced protection, and various COVID-19 vaccine types that are unveiled at a fast pace due to the emergence of SARS-CoV-2 viral variants and sub-variants have all led to a confusing array of vaccination recommendations and schedules from various health authorities. This short review summarises the general principles behind vaccine-induced immunity, newer modified vaccine types such as bivalent vaccines, and the requirements of immune boosting promulgated by various authorities.
Such information would be of practical value to the whole dental team to maintain protection against an endemic disease that may persist for many years into the future.
Clinical Relevance
To describe the mode of action and the types of the current COVID-19 vaccines, and describe their immunogenicity, durability of immunity as well as the immune boosting requirements.
Introduction
In previous articles of this series, we described in detail the various types of newly introduced COVID-19 vaccines, how they work to protect the vaccinees and a number of related issues such as their immunogenicity and side-effects1,2. A major unknown we highlighted at the time was the durability of the vaccine-induced immunity and the need for booster COVID-19 vaccines, if any.
Almost two years after the advent of the COVID-19 vaccines, it is now clear the effective immune response evoked by the multiplicity of available vaccines is relatively short-lived and hence needs periodic boosting at defined intervals. Nevertheless, many surrounding issues related to so called, vaccine boosting are still uncertain.
Front line health care workers including dental professionals should be knowledgeable of the current information on COVID-19 vaccination procedures and the newer vaccine types such as the monovalent and bivalent vaccines, not only to protect themselves and the dental team from a relentlessly evolving endemic infection, but also to advise their patients and the wider public. Here. we attempt to provide the profession with a summary of the current data on COVID-19 vaccination regimentation and schedules, vaccine immunogenicity and durability of protection.
The information below mostly appertains to mRNA vaccine strains but is broadly applicable to most of the other approved vaccine strains currently in use. It should be further emphasised that the information was current at the time of writing (September 2, 2022). However, as this is a rapidly evolving field, readers are advised to keep abreast of the emerging new data through referral to authoritative texts such as the `Green Book` (particularly Chapter 14a) 3 on COVID-19 and/or the various periodic promulgations by the regional /local dental associations, and the Centres for Disease Control and Preventions (CDC) in the USA.
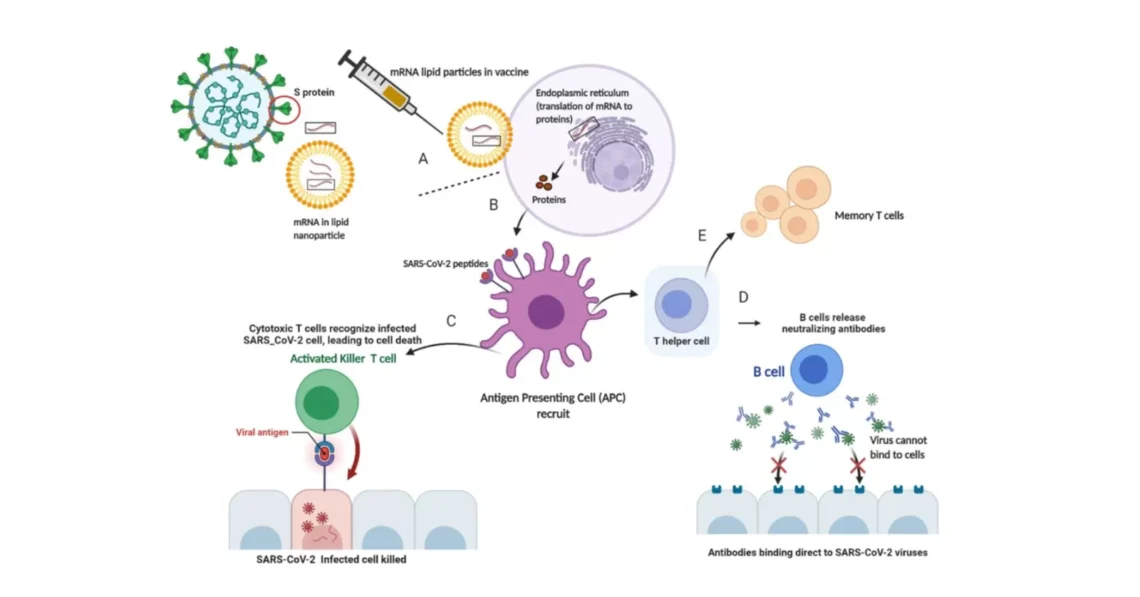
A refresher on Vaccine-induced Immunity
First, a summary of the mechanisms underlying vaccine-induced immunity and the reasons for breakthrough infections (Figure 1).
The aim of a vaccine is to stimulate the body’s own protective immune response, mainly antibody-producing B cells and the enabling T cells, so that, when an individual encounters a specific viral pathogen, then the immune system can quickly recognise and destroy the invading pathogen and terminate the disease process
As for the COVID-19 vaccines the goal is to produce antibodies against the spike (S) proteins on the receptor binding domain (RBD) on the viral surface. These S proteins initiate viral attachment to the susceptible human cells, permitting their entry and then hijacking the DNA of the cell, to produce a viral progeny that will re-infect other cells and cause the infection. Neutralising the critical functionality of the protruding spikes, which facilitate viral entry, with the vaccine-induced, preformed, antibodies, and backed by the enabling T cells, abort the infection.4
Neutralising (NAb), and non-neutralising antibodies (nNAb)
Antibodies produced by the B cells of the immune system can be divided into two basic types: neutralising (NAb), and non-neutralising antibodies (nNAb).The antibodies that block entry of the pathogen into the host cells, and stop the infection are called neutralising antibodies, and should be distinguished from binding antibodies or non-neutralising antibodies (nNAB).
As the name implies, the latter binds to the pathogen, but does not interfere with viral entry into the host cells, possibly because they do not bind to the correct region of the virus. The holy grail of the vaccine manufacturers, therefore, is to produce neutralising antibodies, and not binding antibodies. The current COVID-19 vaccines all are extremely effective in producing these neutralising antibodies to the parental strains of the virus and not particularly to the newer variants such as Omicron and its sub-variants BA.2 and BA.5.
A high frequency of mutations, in our case the spike (S) protein of SARS-CoV-2, through antigenic `shifts` and antigenic `drifts` lead to the emergence of vaccine resistant, recalcitrant strains, as we are now witnessing in the case of COVID-19 pandemic. These, such as Beta, Delta and Omicron variants of SARS-CoV-2 were able to cause breakthrough infection even in the fully vaccinated.
Effective and sterilising immunity
The neutralising antibody response can also be sub-categorised into two different types, the effective and the sterilising antibody response.4 There is a critical difference between these, as effective immunity prevents vaccinee from contracting the illness and he/she may have an asymptomatic infection and become a silent carrier of the disease over a period of time. Consequently, the vaccinee may unknowingly become a `silent spreader` of SARS-CoV-2.
On the contrary, in the case of sterilising immunity the very high level of seroconversion completely aborts the virus multiplication and prevents the further transmission of the virus to another.
<< Back to Contents Menu
EDITOR’S PAGE | MESSAGE FROM THE CHAIR | NEWS | PRODUCTS | FEATURE ARTICLES | CLINICAL | Q&A | EXHIBITIONS & CONFERENCES | SLEEP APNOEA
It should however be noted that vagaries of seroconversion akin to those seen in COVID-19 vaccinees are relatively common. Indeed, there are many previous examples of vaccine precedents for epidemic-prone diseases, such as measles, polio, and hepatitis B, where the vaccination process does not produce sterilising immunity.
Breakthrough infections and immunologic escape
Although vaccines, in general, are highly effective in preventing infections they rarely abort an infection completely. The only exception perhaps is the smallpox that has been vanquished from the face of the earth through a successful vaccination programme in the last century. One major reason for the poor effectiveness of some of the current vaccines, apart from the natural waning of immunity associated with any vaccination procedure (Figure 2) is the behaviour of different viruses and the frequency of their antigenic mutations and switching.
A high frequency of mutations, in our case the spike (S) protein of SARS-CoV-2, through antigenic `shifts` and antigenic `drifts` lead to the emergence of vaccine resistant, recalcitrant strains, as we are now witnessing in the case of COVID-19 pandemic. These, such as Beta, Delta and Omicron variants of SARS-CoV-2 were able to cause breakthrough infection even in the fully vaccinated. This phenomenon with lower and relatively short-lived protection in the fully vaccinated called `immunologic escape` of the virus, led to enhanced infection transmissibility, with concomitant recurrent new waves of infection throughout the world.
To make matters worse, in 2022, sub-variants of Omicron such as BA.1, BA.2, BA.2.12.1, BA.4, and BA.5 emerged and these, with very high infectivity relative to the ancestral strain, appear to be the most antigenically divergent variants of SARS-CoV-2 to date. The new sub-variants rapidly outcompeted their predecessors and infected population with either pre-existing population immunity from vaccination, infection, or both. To date the latter variants are the major agents of COVID-19 illnesses and deaths.5 It is noteworthy however, that breakthrough infections in the vaccinated are relatively mild and significantly minimise severe infection and death.6

Vaccine types: The monovalent and bivalent vaccines
As mentioned, the incessant emergence of SARS-CoV2 variants has led to the development of vaccines for viral variants by a multiplicity of vendors with the objective of attaining a better match in vaccine-induced immunity against the circulating old and the emerging new viral strains. These newer vaccines, termed bivalent vaccines currently available or are being released in UK and USA, as opposed to the original monovalent vaccine against the ‘wild-type’ (also called `ancestral`) strains, contain two different antigens. Each antigen is based on a different SARS-CoV2 strain, and hence broadening vaccine induced-immunity with the likelihood of providing better protection against the targeted variants of SARS-CoV2. The bivalent ‘wild-type’/Omicron BA.1 vaccine, is likely to be available for use in the UK in the autumn of 2022, whereas the Bivalent ‘wild-type’/Omicron BA.4 or 5 is already available in USA7 and yet to be approved in the UK.
Booster Vaccinations and Boosting
There is ample data to indicate that the immunogenicity and hence the protection conferred by a full, three-dose vaccination schedule of either the monovalent or bivalent COVID -19 vaccines is relatively short-lived. Yet, as mentioned, they are highly effective against serious illnesses (hospitalisation and death). Hence booster vaccinations have been recommended by various authorities in many jurisdictions including the UK.
In the case of the Pfizer-BioNTech mRNA COVID-19 Vaccine a single booster dose, at least six months, but preferably 3 months, after completion of the primary series has been recommended. In the UK the advice from BDA which follows guidance from the Joint Committee on Vaccination and immunisation (JCVI) is to offer a COVID-19 booster vaccines to a number of groups such as frontline health care workers including dental care workers.8
JCVI also advises that the timeliness of vaccination is more important than the type of booster vaccine used. The authority states that the key priority of the autumn programme is for `eligible individuals to be offered a booster vaccine dose to increase their immunity against severe COVID-19 ahead of winter 2022 to 2023`. JCVI states that a UK-approved booster vaccine (such as a monovalent Original ‘wild-type’ mRNA vaccine) should be an alternative if the bivalent vaccines are not available. This alternative is better than not being boosted. The autumn booster vaccine dose is recommended to be taken preferably at least 3 months after the previous dose.8
The following addresses in detail the COVID-19 boosters and issues related to boosting.
Booster types
Essentially the following (Moderna mRNA) vaccine types could be used for booster vaccination depending on their availability in a specific jurisdiction or locality:
It is clear, from the current growing database on vaccine efficacy that even the original vaccines against the ancestral strains of SASR-CoV-2 still produce excellent protection against severe disease, so the imperative should be to be vaccinated, with either the original monovalent or the newer bivalent vaccine so as to mitigate severe illness.
Monovalent vaccines
- Moderna mRNA original “wild-type” vaccine
- Pfizer-BioNTech mRNA (Comirnaty) original “wild-type” vaccine
- Novavax Matrix-M adjuvanted wild-type vaccine
Bivalent vaccines
- Moderna mRNA bivalent omicron BA.1/original “wild-type” vaccine (Expected soon in the UK)
- The (Moderna) mRNA bivalent Omicron BA.4 and BA.5/`wild-type’ vaccine (currently available in the USA)
Issues surrounding the booster vaccine administration and bivalent vaccines
Booster vaccine ingredients
The updated bivalent boosters contain mRNA instructions for both the ancestral/wild-type spike (S) protein, and the spike proteins for Omicron BA.1, or BA.4 and BA.59, 10, These spike proteins from variants are extremely similar but not identical, and hence induce a broader immune response. The remaining ingredients of the vaccine such as the lipids, salts and acids, which assist the stability of the mRNA are identical to the original formulation, and so is the overall dose.
Effectiveness of the newer bivalent vaccines
The vaccine authority in the UK, JCVI after reviewing the new data on bivalent vaccines have declared that the neutralising antibody levels generated after vaccination with a bivalent vaccine are only marginally higher than after vaccination with a monovalent (original, wild-type) vaccine. However, the Medicines and Healthcare Products Regulatory Agency (MHRA) of the UK which approved Moderna’s bivalent vaccine for use in adults, has stated that its approval was based on data showing that the bivalent vaccine triggered a “strong immune response” against both BA.1 and the original virus, as well as a “good immune response” against BA.4 and BA.5. Further large, population-based, head-to-head trials are awaited to confirm these findings. Indeed, a number of clinical trials of these newest formulations are still underway, and many scientists contend that these studies will provide ample evidence of superior effectiveness of bivalent than the monovalent vaccines10,11.
Vaccine Safety
Due to the many millions of the original monovalent vaccines delivered throughout the World, some contend that no new data are required to assert the safety of the bivalent vaccines. Nevertheless, available data from various clinical trials of the bivalent booster versions show that they have a risk profile very similar to the original vaccines.
Boosting those who are recently infected
Individuals who have been infected with Omicron or other variants retain a high degree of immunity to the virus immediately after infection. However, their antibody levels and hence the degree of protection against the disease decline soon afterwards. Hence, according to US authorities they suggest boosting antibody levels to confer additional protection with a monovalent or preferably a bivalent booster vaccine preferably three months post-infection.
Concluding remarks
Achieving durable immunity from COVID-19 vaccines is one of the biggest current challenges the community at large is facing. Until then it is likely that dental care workers will require booster COVID-19 vaccinations periodically, akin to the annual seasonal influenza vaccinations. However, the periodicity of COVID-19 vaccination administration for the future, whether six-monthly or annual, is still uncertain, until further data emerge. Six monthly schedules are the preferred current option by almost all authorities. Additionally, whether the current versions of the vaccines will be effective against the putative new variants are other great uncertainties that may dictate the frequency of vaccine administration.
If there are minor variations in the Omicron viral spike proteins (antigenic drifts) then it is likely that the current bivalent vaccines will be effective against such potential new variants. However, in the event of a significant major change in the antigenic composition of the spike proteins (antigenic shift) then there will possibly be a need for a completely new vaccine strain rather than a hybrid cocktail type vaccine. It is clear, from the current growing database on vaccine efficacy that even the original vaccines against the ancestral strains of SASR-CoV-2 still produce excellent protection against severe disease, so the imperative should be to be vaccinated, with either the original monovalent or the newer bivalent vaccine so as to mitigate severe illness.
The COVID-19 vaccine story is still unfolding and there are some silver linings on the horizon that bodes well for the future. The current COVID-19 vaccines are all designed for immunisation via the intramuscular (IM) route. The high frequency of breakthrough infections in the vaccinees we are currently witnessing implies that the IM route may not be the ideal route for COVID-19 vaccine delivery as SARS-CoV-2 portal of entry is essentially through the upper respiratory tract. Hence breakthrough infections after IM vaccination may be a result of the low degree of mucosal immunity generated in the upper airways. To redress this issue, newer vaccine delivery modes that confer mucosal immunity of the upper respiratory tract are currently being successfully trialled. These include the `user-friendly` vaccines, currently in Phase II and III trials that are delivered by the nasal route through a fine spray or drop instillation12. There is new, comforting data to indicate that the novel nasal vaccines are likely to be superior to the current injectable type vaccines due to their efficacy as well as the ease and simplicity of delivery and administration13.
Another novel approach to vaccine production is called `deep mutational scanning` where the surface antigen `drifts and shifts` of the virus could be investigated in silico using artificial intelligence and machine learning.14 This means that vaccines for the next viral variant or indeed a new pathogen could be predicted far in advance, and the infection annihilated in the bud, prior to becoming a ravaging pandemic.
These novel developments in COVID-19 vaccinology may not only simplify the current strict vaccination protocols and regimentation required for the frontline health care workers, to keep a vicious virus at bay, but also mitigate future pandemics with emerging new viruses. Indeed, the predicted evolutionary antigenic shifts of SARS-CoV-2 spike proteins that may lead to new virulent variants with unpredictable severity 15.16, could be abrogated by these technical advances in COVID-19 vaccines, thus preventing the anticipated major viral pandemics.
References
- Samaranayake, L.P.; Anil S Understanding COVID-19 vaccines and immunity Dental Update 2021 48(1) 156-159.
- Samaranayake L P , Chang W W, Panduwawala C COVID-19 Vaccines: Vagaries and Vacillations Dental Update 2021 48(4):323-326.
- UK Government (UK.Gov) Green Book https://www.google.com/search?q=Green+book+COVID-19+BDA&rlz=1C5CHFA_enHK710HK711&oq=Green+book+COVID-19+BDA&aqs=chrome..69i57.13604j0j15&sourceid=chrome&ie=UTF-8
- Arashkia A, Jalilvand S, Mohajel N, Afchangi A, Azadmanesh K, Salehi-Vaziri M, Fazlalipour M, Pouriayevali MH, Jalali T, Mousavi Nasab SD, Roohvand F, Shoja Z; SARS CoV-2 Rapid Response Team of Pasteur Institute of Iran (PII). Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects. Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. PMID: 33594794; PMCID: PMC7646037.
- Deb P, Molla MMA, Saif-Ur-Rahman KM, Das MC, Das D. A review of epidemiology, clinical features and disease course, transmission dynamics, and neutralization efficacy of SARS-CoV-2 variants. Egypt J Bronchol. 2021;15(1):49. doi: 10.1186/s43168-021-00090-x. Epub 2021 Nov 6. PMCID: PMC8571979.
- Hall V, Foulkes S, Insalata F, Kirwan P, and SIREN Study Group. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022 Mar 31;386(13):1207-1220. doi: 10.1056/NEJMoa2118691. Epub 2022 Feb 16. PMID: 35172051; PMCID: PMC8908850.
- Mahase: E Covid-19: UK will roll out Moderna’s omicron BA.1 vaccine as part of autumn booster programme BMJ 2022;378:o2038http://dx.doi.org/10.1136/bmj.o203
- UK Government: Interim statement https://www.gov.uk/government/publications/jcvi-interim-statement-on-covid-19-autumn-2022-vaccination-programme/joint-committee-on-vaccination-and-immunisation-jcvi-interim-statement-on-the-covid-19-vaccination-programme-for-autumn-2022#fn:
- Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against covid-19. medRxiv [preprint] 2022 (published online) https://www.medrxiv.org/content/10.1101/2022.06.24.22276703v1.full.pdf: 10.1101/2022.06.24.22276703 25). doi:10.1101/2022.06.24.22276703. https://www.medrxiv.org/content/10.1101/2022.06.24.22276703v1.
- Fang, Z. & Chen, S. (2022) Bivalent mRNA vaccine booster induces robust antibody immunity against Omicron subvariants BA.2, BA.2.12.1 and BA.5. bioRxiv. doi:10.1101/2022.07.19.500616. https://www.biorxiv.org/content/10.1101/2022.07.19.500616v1.
- Summary of product characteristics: Spikevax bivalent Original/Omicron. Gov.uk. 2022.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/ file/1097996/Spikexax_bivalent_Original_Omicron_SmPC.pdf
- Alu A, Chen L, Lei H etal Intranasal COVID-19 vaccines: From bench to bed Lancet 76, 103841, 2022 DOI:https://doi.org/10.1016/j.ebiom.2022.103841
- WHO’s landscape of COVID-19 vaccine candidates. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines, 24-November 2021.
- Fowler DM, Fields S. Deep mutational scanning: a new style of protein science. Nature Methods 2014;11(8):801-07.
- Markov, P. V., Katzourakis, A. & Stilianakis, N. I. Antigenic evolution will lead to new SARS-CoV-2 variants with unpredictable severity. Nat. Rev. Micobiol. 2022, 20, 251–252. 26.
- Samaranayake L, Fakhruddin KS. Pandemics past, present, and future: Their impact on oral health care. J Am Dent Assoc. 2021 Dec;152(12):972-980. doi: 10.1016/j.adaj.2021.09.008. Epub 2021 Nov 6. PMID: 34749921; PMCID: PMC8570943.
The information and viewpoints presented in the above news piece or article do not necessarily reflect the official stance or policy of Dental Resource Asia or the DRA Journal. While we strive to ensure the accuracy of our content, Dental Resource Asia (DRA) or DRA Journal cannot guarantee the constant correctness, comprehensiveness, or timeliness of all the information contained within this website or journal.
Please be aware that all product details, product specifications, and data on this website or journal may be modified without prior notice in order to enhance reliability, functionality, design, or for other reasons.
The content contributed by our bloggers or authors represents their personal opinions and is not intended to defame or discredit any religion, ethnic group, club, organisation, company, individual, or any entity or individual.

